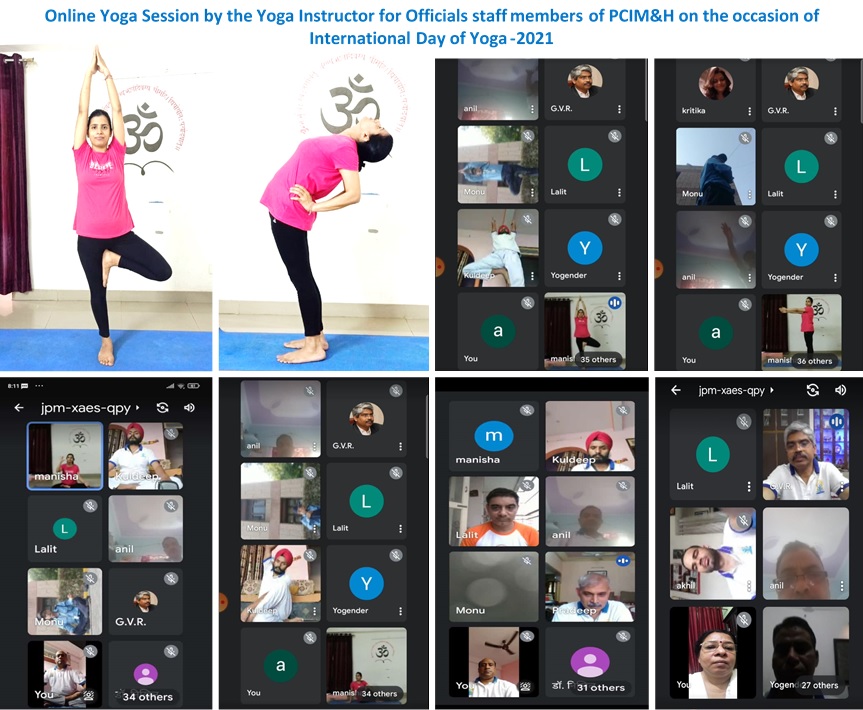
Director's Message

Dr. Raman Mohan Singh
PCIM&H was initially established as PCIM in the year 2010 and later in pursuance to the decision of Central Government dated 20th March 2014,... Read More
Contact Us
Pharmacopoeia Commission for Indian Medicine & Homoeopathy (PCIM&H)
Kamla Nehru Nagar, Post Kavi Nagar
Ghaziabad, Uttar Pradesh
India
Pincode-201002
E-mail: dir.pcimh-ayush[at]gov[dot]in
Telephone: +91-120-2787014